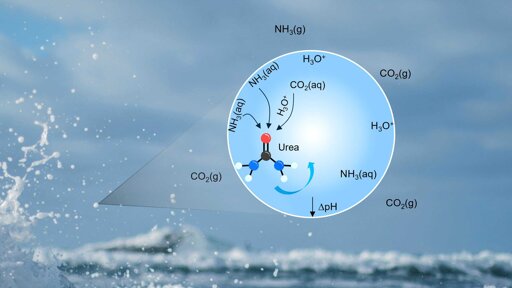
Urea is considered a possible key molecule in the origin of life. ETH researchers have discovered a previously unknown way in which this building block can form spontaneously on aqueous surfaces without the need for any additional energy.
Urea is one of the most important industrial chemicals produced worldwide. It is used as a fertilizer, for the production of synthetic resins and explosives and as a fuel additive for cleaning car exhaust gases. Urea is also believed to be a potential key building block for the formation of biological molecules such as RNA and DNA in connection with the question of the origin of life.
Signorell’s team studied tiny water droplets such as those found in sea spray and fine mist. The researchers observed that urea can form spontaneously from carbon dioxide (CO₂) and ammonia (NH₃) in the surface layer of the droplets under ambient conditions. The physical interface between air and liquid creates a special chemical environment at the water surface that makes the spontaneous reaction possible.